Editor’s note: David Bennett died March 8, 2022, two months after receiving a pig heart through a transplant described below. He lived longer with an animal heart than any human before.
The transplant team from the University of Maryland School of Medicine (UMSOM) kept doing everything right: Time and again they transplanted a heart from a pig that had been genetically modified so that the organ would not be rejected by the baboon that received it. Sure enough, the baboons’ immune systems did not attack the hearts. The grafts on the arteries held, and the hearts pumped blood — for about 48 hours.
Then the hearts slowly stopped beating. “It was like they were running out of gas,” recalls Muhammad Mohiuddin, MBBS, the lead surgeon-scientist on the team and director of the cardiac xenotransplantation program at UMSOM in Baltimore.
The xenotransplantation team learned that doctors performing the same procedures in Germany were keeping the pig organs in the XVIVO Heart Box — a machine that uses a special solution to preserve each heart until the moment comes to implant it. When the UMSOM team started using that process three years ago for its pig heart transplants, Mohiuddin says, the baboons lived for up to nine months.
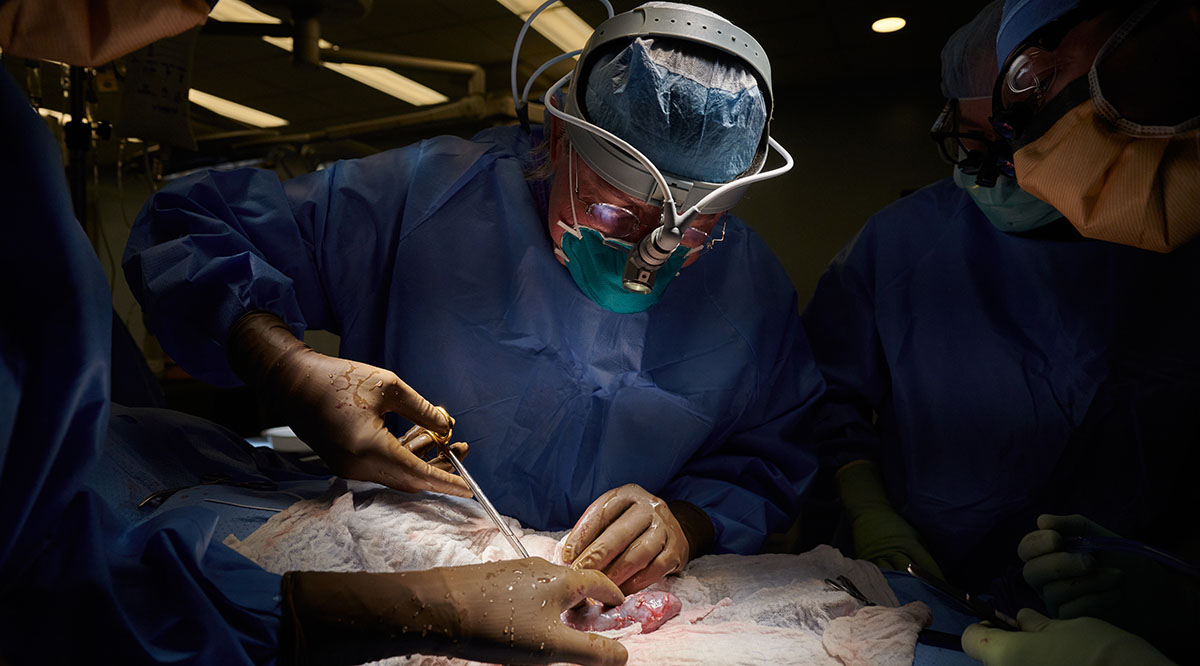
That is why 57-year-old David Bennett lives today with a pig heart in his chest — having become the first living person to receive a genetically modified animal heart through a surgery last month at the University of Maryland Medical Center.
After decades with little public attention, the quest to transplant animal organs to humans — a form of xenotransplantation — seems to have suddenly made stunning strides. In recent months, NYU Langone Health in New York City and the University of Alabama at Birmingham (UAB) Marnix E. Heersink School of Medicine (SOM) announced a total of three transplants of pig kidneys into people who had been declared brain dead. The kidneys functioned until the planned end of the experiments: 54 hours at NYU Langone (twice) and 77 hours at UAB Heersink SOM.
These historic procedures happened because of countless under-the-radar experiments that were carried out at medical school labs and university hospitals over the past 20-plus years to overcome obstacles that had repeatedly caused organs transplanted from one animal to another to fail. The emerging science of gene editing, among other advances, recently enabled scientists to circumvent most of those obstacles.
“What we're seeing is a culmination of decades of research and work that, in large part, was done behind the scenes,” says Parsia Vagefi, MD, chief of the Division of Surgical Transplantation at UT Southwestern Medical Center in Dallas, who has conducted studies on transplants between pigs and nonhuman primates.
This progress could eventually lead to lifesaving procedures for the more than 100,000 people in the United States who, according to the federal government, are on waiting lists for organ transplants. Not nearly enough organs are donated to meet the demand.
“We see patients [with organ failure] five days a week in clinic, and I know that the majority of those patients are going to die before they ever get an offer for a transplant,” notes Jayme Locke, MD, MPH, who led the recent xenotransplant at UAB Heersink SOM. “To not have enough for everyone who needs one is the worst feeling.”
Overcoming obstacles
Attempts in the early 1900s to transplant animal organs and tissue to humans came to a halt because the human immune system invariably identified the material as an invader and attacked it, according to a history of cross-species transplantation. Then from the 1960s through the 1990s, doctors used immunosuppressive drugs when transplanting livers, kidneys, and hearts from chimpanzees and baboons to people, sometimes with moderate success: The survival times ranged from two hours to several weeks to nine months, reports David Cooper, MBBS, PhD, a transplant research surgeon at Massachusetts General Hospital in Boston.
Meanwhile, transplants among animals produced slow progress, with survival of organs advancing by days or weeks with each experiment, Vagefi says.
“In the early 2000s, we had a nonhuman primate live for three months with a pig kidney,” he recalls. “Back then, that was an amazing feat.”
Several developments moved the field forward.
During the 1990s, pigs replaced nonhuman primates as the preferred source of organs for xenotransplantation experiments, according to Robert Montgomery, MD, PhD, director of the NYU Langone Transplant Institute and the lead surgeon on the recent xenotransplants there. Among the reasons: They have short gestation periods and produce large litters, their organs are close in size to those of humans, they are less likely than nonhuman primates to transfer zoonotic diseases to humans (because of genetic differences), and their tissues (such as heart valves and skin grafts) have been successfully transplanted into people for years.
One problem, however, is that pigs, like most mammals, have a sugar molecule called alpha-gal that humans (and some nonhuman primates) do not. The human immune system produces a severe immune reaction to alpha-gal, leading the body to reject the transplanted organ.
Researchers tried several ways to overcome that and other challenges, including editing genomes to knock out alpha-gal. While scientists have been editing genomes since the early 1990s, the development of new technologies — particularly the CRISPR-Cas9 tool in 2012 — revolutionized the process. As explained previously by AAMCNews, the tool uses programmable RNA and an enzyme, Cas9, to locate specific genetic sequences in an organism and cut the DNA’s double helix so that sequences can be deleted, added, or replaced. That capability makes editing faster, easier, and more precise, Montgomery notes.
These editing technologies enabled scientists to create pigs with organs that were less likely to be attacked by primates’ immune systems. The key edit was knocking out the alpha-gal antigen. In some pig lines, scientists added nine more edits: removing two other antigens that set off immune responses, removing a gene that would allow excessive organ growth inside recipients, and adding six human genes that foster immune acceptance of pig organs.
For the past several years, researchers have been transplanting organs from such modified pigs into nonhuman primates, with the organs often functioning well for more than six months and sometimes for over a year, Vagefi says.
Success with humans
The increased success in transplanting organs among animals has led researchers to feel that those experiments were becoming, in Montgomery’s words, “an exercise in diminishing returns. There’s only so much you can learn from animal models.”
For one thing, it’s extremely difficult to keep the transplant recipients healthy even if the transplants aren’t rejected. The animals cannot be cared for in the equivalent of a human hospital, they and their human observers cannot communicate well about symptoms, and they can’t follow a doctor’s plan for how to care for themselves.
“They get infected easily. They don’t cooperate,” Montgomery says. “You have to put them under an anesthetic to draw blood. It’s all very difficult.”
In addition, “the nonhuman primates, while similar to us, are immunologically distinct from humans,” notes Locke, director of the Comprehensive Transplant Institute at UAB Heersink SOM.
Locke says she couldn’t be sure how a human’s immune system would respond to a pig kidney until she gave a human a pig kidney. In addition, she notes that the mean arterial blood pressure in humans is higher than in pigs and nonhuman primates. Would the transplanted organ’s vascular system hold up to that pressure?
“It’s a big jump to go from primates to humans,” Montgomery adds. “There have been a lot of [medical interventions] that looked promising in nonhuman primates, but humans had a different response.”
NYU Langone and UAB Heersink SOM were not ready to start clinical trials, which involve human patients and would require approval by the Food and Drug Administration (FDA). Rather, their researchers pursued an experiment that they don’t believe has been tried before: transplanting an organ into a person who had been declared brain dead, with machines keeping the bodily functions working.
“We wanted to be able to test this procedure without risking a living person,” Locke says.
“Brain death” is not the same as “vegetative state.” As explained by Johns Hopkins Medicine, someone in a vegetative state has a functioning brain stem and might recover, while brain death is the “irreversible cessation of all functions of the entire brain. … A person who is brain dead is dead, with no chance of revival.”
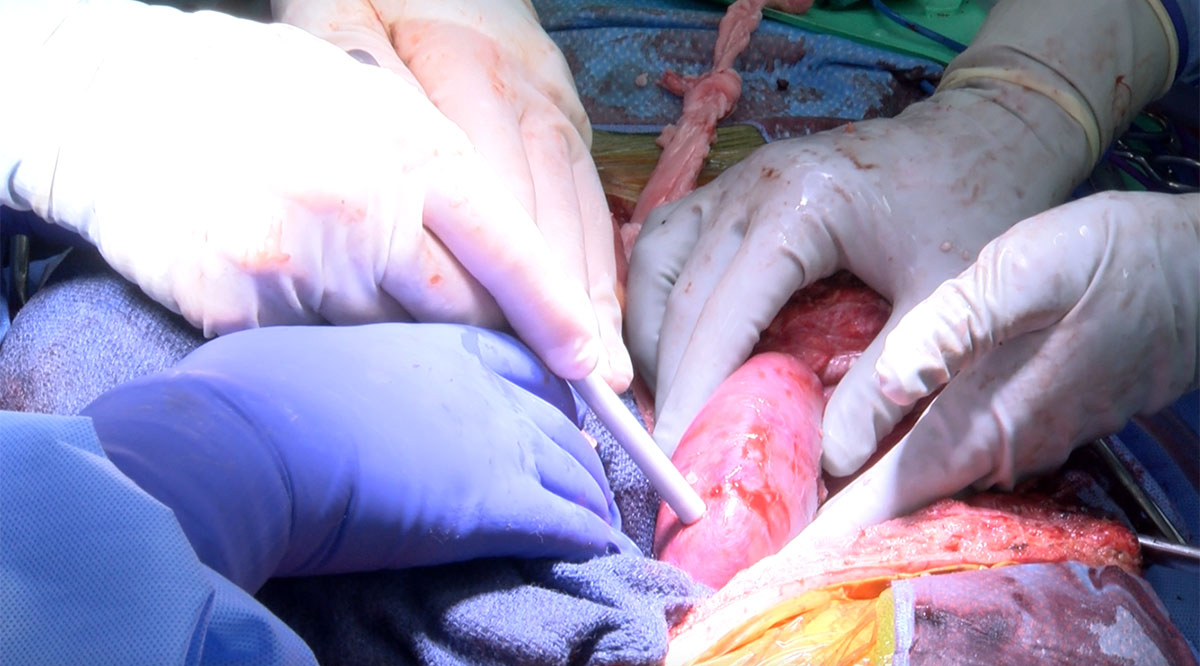
NYU Langone and UAB Heersink SOM found families who were willing to donate the bodies of brain-dead loved ones for the experiments last fall. In two surgeries about two months apart, the team at NYU Langone attached kidneys from pigs with alpha-gal removed to blood vessels in the upper legs of two patients, so that the team could easily monitor the organs and take tissue samples. Between those two surgeries, the team at UAB Heersink SOM replaced a person’s two kidneys with porcine kidneys. (UAB Heersink SOM and UMSOM, which did the subsequent heart transplant, used pigs with the alpha-gal and nine more genetic edits.)
Montgomery describes a sense of heavy anticipation after the kidneys were attached: “I said to the other surgeons around the table, right before we took the clamps off, ‘I don't know what’s going to happen here today. But whatever happens, we’re going to learn something from it, and it’s going to be important.’”
NYU Langone and UAB Heersink SOM reported that the kidneys immediately turned pink as they filled with blood and that they produced urine. They said the humans’ immune systems did not appear to attack the new organs and that the blood vessels held.
At the University of Maryland Medical Center, Bennett continues recovering with a pig heart — a heart that had been acquired for transplant into a nonhuman primate as part of the school’s research. After doctors there learned that Bennett was out of options to treat his failing heart — having been rejected for a human heart transplant — they received emergency authorization from the FDA to conduct the porcine transplant in early January through its compassionate use provision for experimental treatments.
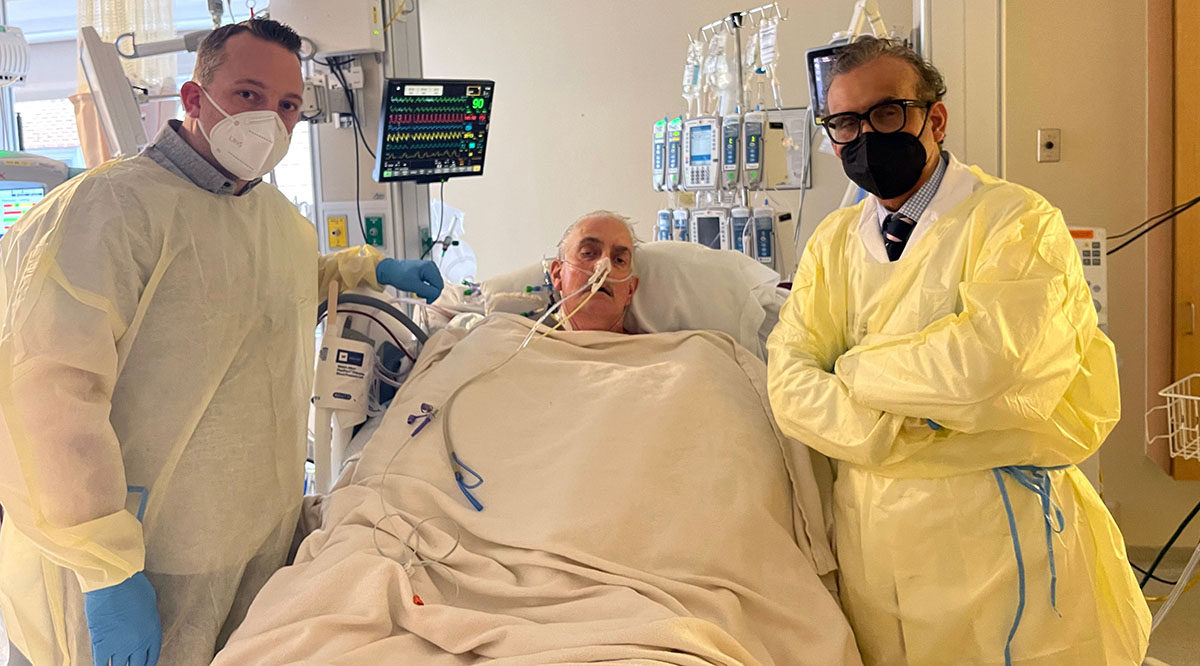
“I never imagined doing this on a person during my lifetime,” says Mohiuddin, who was on the transplant team. “When you hear that heartbeat — even in the baboon, it’s a great feeling, but nothing compares to seeing a heart beat in a human.”
Next steps
The institutions plan to continue transplant experiments among animals and brain-dead humans to build more data to use in applications for clinical trials, possibly within two years. Among the major questions for those trials: Will the transplanted organs last for many years or just long enough for a human organ to become available?
At Mass General, Cooper contends that there’s already enough evidence from animal-to-animal transplants to justify a small, phased trial of pig kidney transplants to humans. Transplants in brain-dead people don’t provide useful information, he says, because bodily functions after brain death deteriorate rapidly and make it impossible to assess the impact of transplanted organs. He believes that observations over just several days yield no new insights.
Locke, however, says that watching the kidneys spring to life in a person gave her a surge about new possibilities: “There was a pause right before removing the clamps: I looked up at my partners and we all had this silent understanding of the magnitude of the moment.
“When the clamps came off, there was this sense of absolute relief and yet pure joy. Wow, this is hope. We actually might be able to walk into clinic and have a cure for every single person I see.”
